Study 046:
IXEMPRA in combination
with capecitabine
STUDY OBJECTIVE
To evaluate the safety and efficacy of IXEMPRA® (ixabepilone) plus capecitabine vs capecitabine alone in metastatic or locally advanced breast cancer resistant to both an anthracycline and a taxane.1
Study design and baseline characteristics
Study 046 design1,2
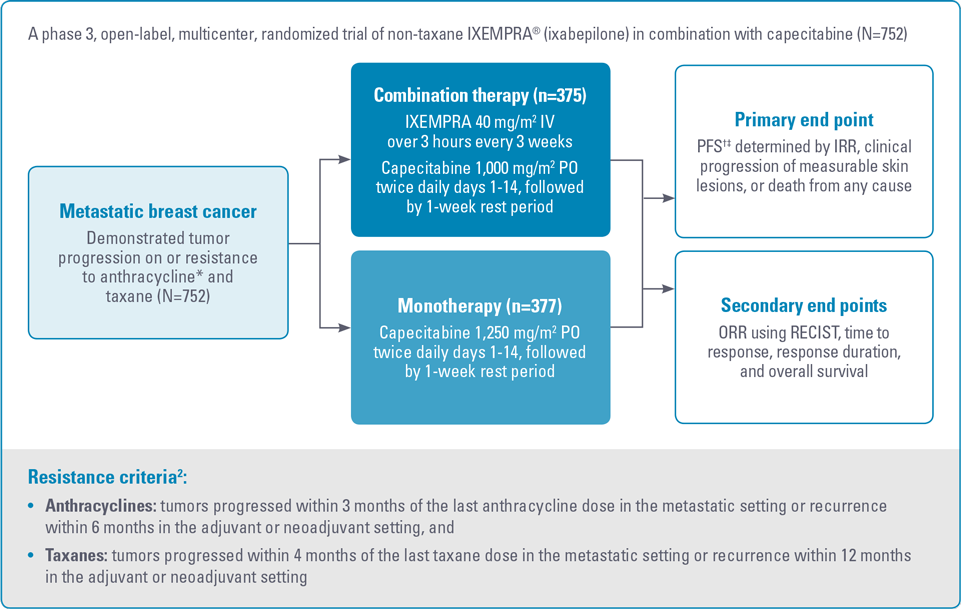
IRR, independent radiologic review; ORR, objective tumor response rate; PFS, progression-free survival; PO, by mouth; RECIST, Response Evaluation Criteria in Solid Tumors.
* For anthracyclines, patients who received a minimum cumulative dose of 240 mg/m2 of doxorubicin or 360 mg/m2 of epirubicin were also eligible.2
† PFS was defined as time from randomization to radiologic progression, based on IRR of intent-to-treat population.1,2
‡ Patients were censored for PFS at the last date of tumor assessment prior to the start of subsequent therapy. In patients where independent review was not available, PFS was censored at the randomization date.2
Study 046 baseline patient demographics and clinical characteristics1,2
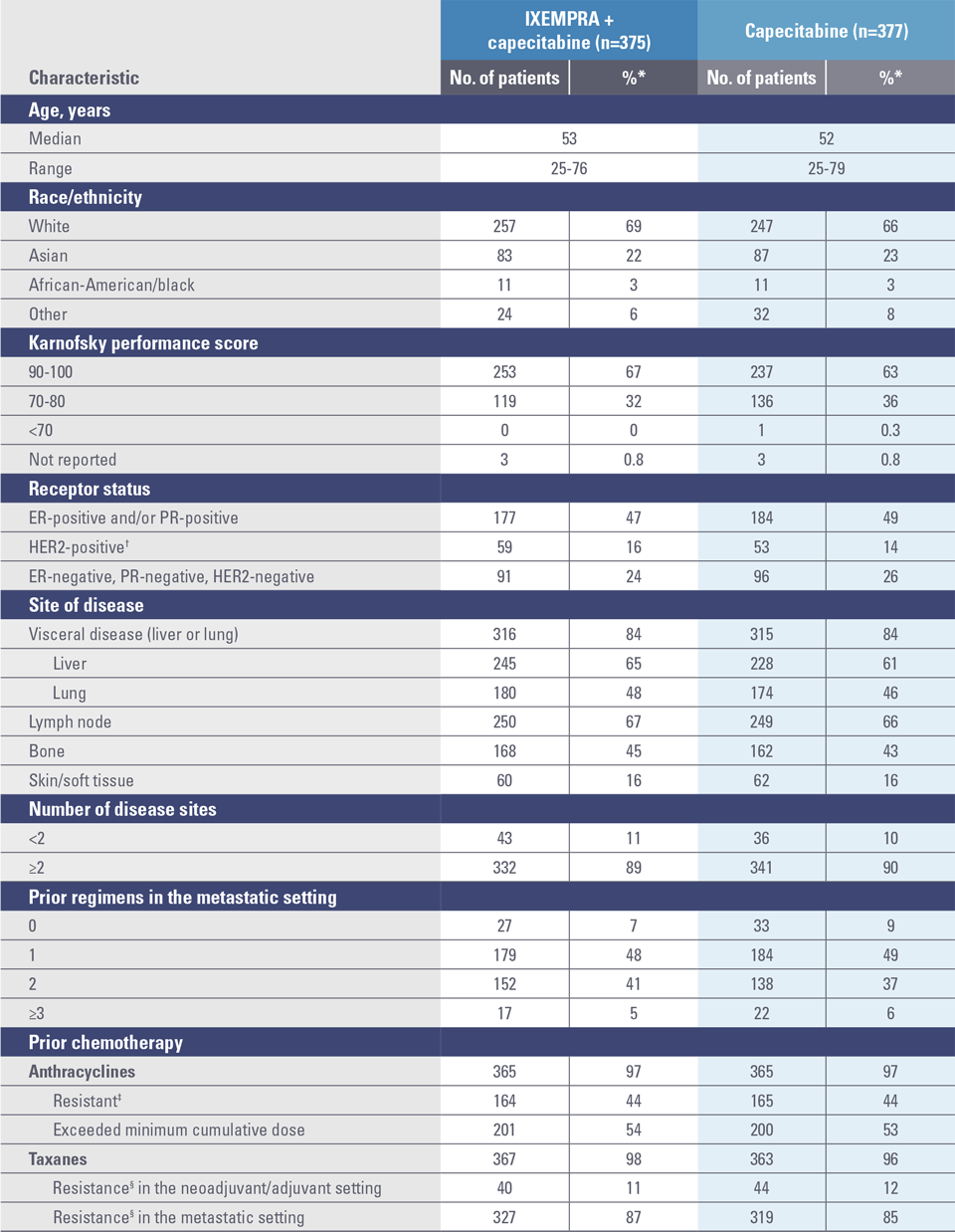
ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; PR, progesterone receptor.
* Percentages may not add to 100% due to rounding and/or unknown data for some patients.1
† Defined as positive by fluorescence in situ hybridization or 3+ by immunohistochemistry.1
‡ Resistance is defined as recurrence within 6 months in the adjuvant or neoadjuvant setting or progression within 3 months of the last anthracycline dose in the metastatic setting.1
§ Resistance is defined as recurrence within 12 months in the adjuvant or neoadjuvant setting or progression within 4 months of the last taxane dose in the metastatic setting.1
In combination with capecitabine, IXEMPRA® (ixabepilone) significantly improved progression-free survival (PFS) and more than doubled the objective tumor response rate (ORR) vs capecitabine alone2
IXEMPRA in combination with capecitabine demonstrated statistically significant improvement in PFS vs capecitabine alone in patients with metastatic or locally advanced breast cancer whose tumors were resistant to an anthracycline (or for whom further use was contraindicated) and a taxane2
Study 046 results (N=752)2
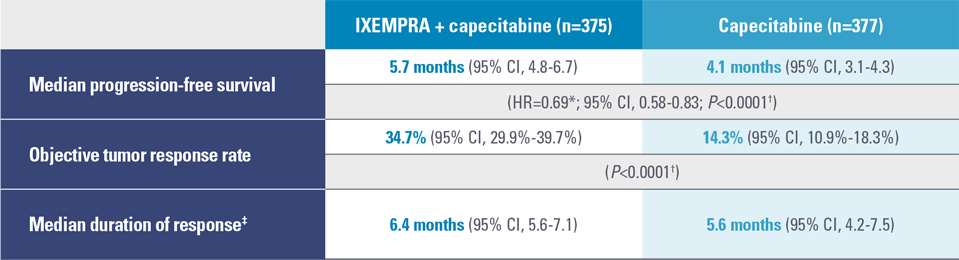
CI, confidence interval; HR, hazard ratio.
* For the hazard ratio, a value less than 1.00 favors combination treatment; CI adjusted for interim analysis.2,3
† Stratified by visceral metastases in the liver/lung, prior chemotherapy in the metastatic setting, and anthracycline resistance.2
‡ Calculated for all patients who achieved an objective tumor response (IXEMPRA + capecitabine, n=130; capecitabine, n=54).3
- Patients received a median of 5 cycles and 4 cycles in the combination and capecitabine groups, respectively2
- In patients who progressed on an anthracycline and a taxane, approximately 70% received IXEMPRA plus capecitabine after initial taxane failure3
- There was a statistically significant improvement in median PFS: 5.7 months vs 4.1 months, respectively (P<0.0001)2
- There was a statistically significant improvement in median ORR: 34.7% vs 14.3%, respectively (P<0.0001)2
- There was no statistically significant difference in overall survival between treatment arms in this and a similarly designed study2
- In the study described above, median overall survival was 12.9 months (95% CI, 11.5-14.2) in the combination therapy arm and 11.1 months (95% CI, 10.0-12.5) in the capecitabine alone arm (HR=0.90; 95% CI, 0.77-1.05; P=0.19)2
- In the second trial comparing IXEMPRA in combination with capecitabine versus capecitabine alone, conducted in 1,221 patients pretreated with an anthracycline and a taxane, the median overall survival was 16.4 months (95% CI, 15.0-17.9) in the combination therapy arm and 15.6 months (95% CI, 13.9-17.0) in the capecitabine alone arm (HR=0.90; 95% CI, 0.78-1.03; P=0.12)2
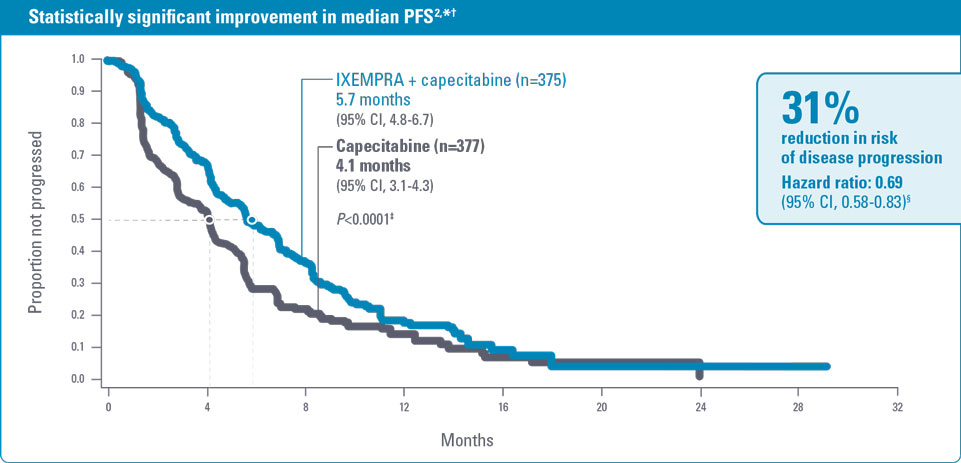
CI, confidence interval; ORR, objective tumor response rate.
* Evaluated using Response Evaluation Criteria in Solid Tumors (RECIST).2
† Stratified by visceral metastases in the liver/lung, prior chemotherapy in the metastatic setting, and anthracycline resistance.2
‡ Calculated for all patients who achieved an objective tumor response (IXEMPRA + capecitabine, n=130; capecitabine, n=54).3
- More than double the ORR in the combination group in patients with metastatic or locally advanced breast cancer as evaluated using RECIST2
- Median duration of response: 6.4 months (95% CI, 5.6-7.1) vs 5.6 months (95% CI, 4.2-7.5), respectively2

Trina
Age: 45 years*
Perimenopausal
DIAGNOSIS
Initial stage
- Palpable mass in the right breast
- Mammography revealed 3.5-cm mass
- Ultrasound-guided biopsy confirmed presence of invasive ductal carcinoma
- Chest X-ray, bone scan, and CT scan were negative for metastatic disease
Receptor status
- ER−, PR−, HER2/neu−
TREATMENT HISTORY
- Underwent a mastectomy with axillary node dissection performed approximately 1 month after initial presentation
- 6 of 12 axillary nodes contained macrometastases, and 1 contained 2-mm micrometastasis (pT2N2)
Adjuvant
- Adjuvant therapy (AC–>T) initiated
- Patient subsequently received radiation therapy
PRESENTATION
General
- 9 months after completion of adjuvant therapy, patient complained of abdominal pain and shortness of breath on mild exertion; unable to work a full day due to severe fatigue
Sites of metastases
- Multiple liver and lung lesions
Pertinent labs
- CT-guided biopsy of one of the liver nodules was consistent with invasive ductal carcinoma
- Receptor status confirmed as ER−, PR−, and HER2/neu−
- LFT (AST/ALT) values were normal
- Neutrophil and platelet counts were within normal limits
FIRST-LINE METASTATIC CHEMOTHERAPY
- 16 months after surgery, patient was started on docetaxel plus capecitabine
- Further anthracycline therapy was contraindicated
- Patient progressed within 4 months
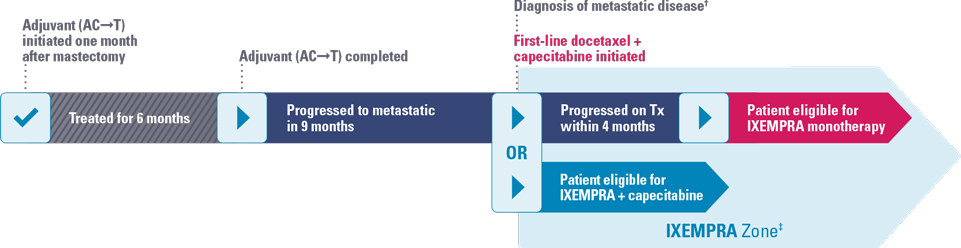

Jennifer
Age: 55 years*
Postmenopausal
DIAGNOSIS
Initial stage
- 2.5-cm mass in the upper outer quadrant of the left breast; mass was dense with abundant speculation
- Stranding around the mass and architectural distortion were also present
- Mass was palpable, shotty, non-tender, and soft; mobile axillary lymph nodes were observed (stage IIA)
Receptor status
- ER+, PR+, HER2/neu−
TREATMENT HISTORY
- Lumpectomy and axillary staging; 2 of 11 axillary nodes contained micrometastases, and 1 contained 3-mm metastasis (stage IIB)
Adjuvant
- Docetaxel + doxorubicin (cumulative dose of 300 mg/m2) + cyclophosphamide; G-CSF support
- Additional doxorubicin therapy was contraindicated
- Patient was started on an aromatase inhibitor and radiation therapy after completion of adjuvant chemotherapy
PRESENTATION
General
- 13 months after completion of TAC, patient complained of persistent back pain that was not relieved despite taking an NSAID
Sites of metastases
- Multiple liver and bone lesions
Pertinent labs
- An MRI scan of patient’s spine showed lytic lesions on L4 and L5
- Subsequent bone scans and CT scan revealed numerous skeletal lesions, as well as 3 hepatic lesions, each measuring 1 x 2 cm
- Liver biopsy confirmed receptor status changed to ER−, PR+, and HER2/neu−
- LFT (AST/ALT) values were normal
- Neutrophil and platelet counts were within normal limits
FIRST-LINE METASTATIC CHEMOTHERAPY
- Started on another taxane therapy (progressed within 3 months of starting therapy)
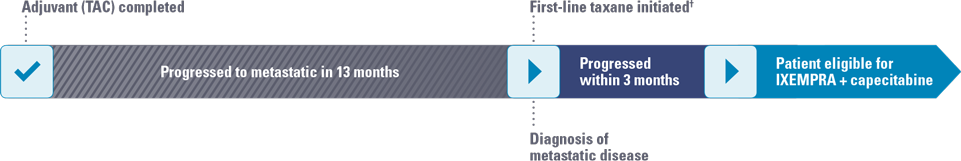
ALT, alanine aminotransferase; AST, aspartate aminotransferase; ER, estrogen receptor; G-CSF, granulocyte colony-stimulating factor; HER2, human epidermal growth factor receptor 2; LFT, liver function test; PR, progesterone receptor; TAC, docetaxel, doxorubicin, and cyclophosphamide.
* Hypothetical patient profile. Photo not of actual patient.
† Further anthracycline therapy was contraindicated.
Safety profile of IXEMPRA® (ixabepilone) in combination with capecitabine
Study 046 peripheral neuropathy2
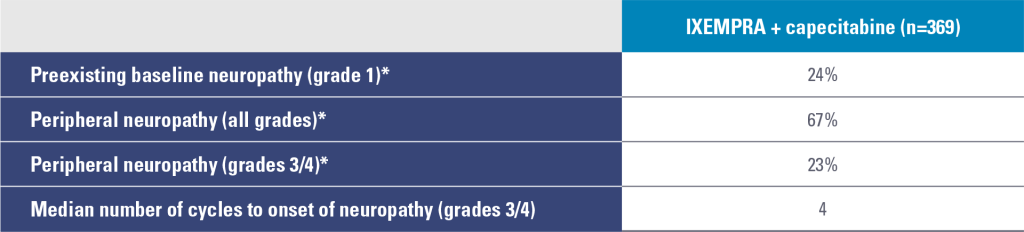
* Sensory and motor neuropathy combined.
- Prior therapy with neurotoxic chemotherapy agents did not predict the development of neuropathy
- Peripheral neuropathy was common and primarily sensory
- Neuropathy is cumulative and generally reversible and should be managed with dose adjustments and delays
- The median time to improvement of grades 3/4 neuropathy to baseline or grade 1 was 6.0 weeks
- Following dose reduction, 80% of patients had improvement or no worsening of their neuropathy
- Discontinuation due to neuropathy was 21%
Study 046 nonhematologic drug-related adverse reactions occurring in ≥5% of patients2
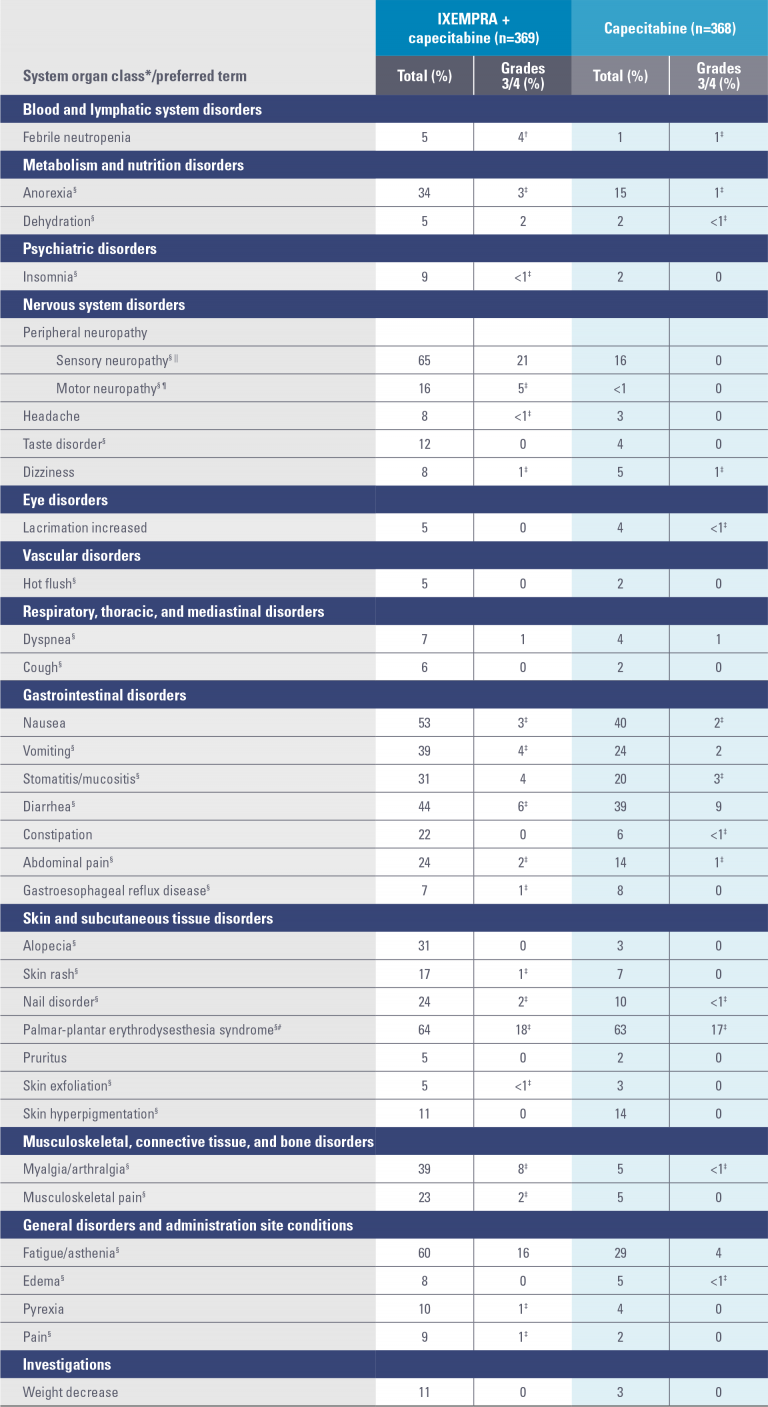
* System organ class presented as outlined in Guidelines for Preparing Core Clinical-Safety Information on Drugs by the Council for International Organizations of Medical Sciences (CIOMS).
† National Cancer Institute Common Terminology Criteria (NCI CTC) grading for febrile neutropenia ranges from grade 3 to grade 5. Three patients (1%) experienced grade 5 (fatal) febrile neutropenia. Other neutropenia-related deaths (9) occurred in the absence of reported febrile neutropenia.
‡ No grade 4 reports.
§ A composite of multiple MedDRA Preferred Terms.
|| Peripheral sensory neuropathy (graded with the NCI CTC scale) was defined as the occurrence of any of the following: areflexia, burning sensation, dysesthesia, hyperesthesia, hypoesthesia, hyporeflexia, neuralgia, neuritis, neuropathy, neuropathy peripheral, neurotoxicity, painful response to normal stimuli, pallanesthesia, paresthesia, peripheral sensory neuropathy, polyneuropathy, polyneuropathy toxic, and sensorimotor disorder.
¶ Peripheral motor neuropathy (graded with the NCI CTC scale) was defined as the occurrence of any of the following: multifocal motor neuropathy, neuromuscular toxicity, peripheral motor neuropathy, and peripheral sensorimotor neuropathy.
# Palmar-plantar erythrodysesthesia (hand-foot) syndrome was graded on a 1-3 severity scale in Study 046.
Study 081:
IXEMPRA monotherapy
STUDY OBJECTIVE
To evaluate the efficacy and safety of IXEMPRA® (ixabepilone) in patients with metastatic or locally advanced breast cancer resistant to an anthracycline, a taxane, and capecitabine.1
Study design and baseline characteristics
Study 081 design1,2
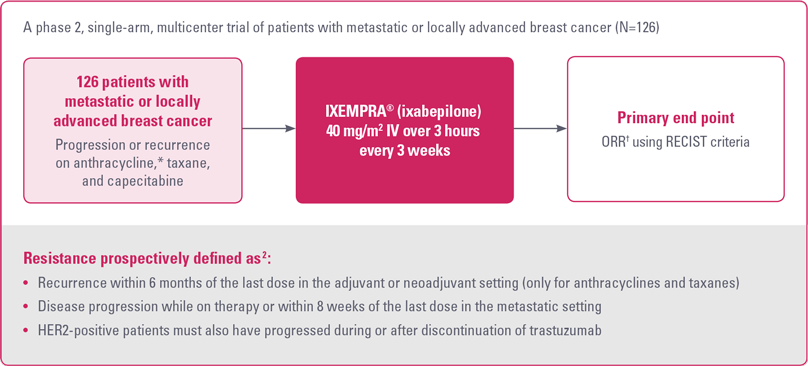
HER2, human epidermal growth factor receptor 2; ORR, objective tumor response rate; RECIST, Response Evaluation Criteria in Solid Tumors.
* For anthracyclines, patients who received a minimum cumulative dose of 240 mg/m2 of doxorubicin or 360 mg/m2 of epirubicin were also eligible.2
† As determined by independent radiologic review.2
Study 081 baseline patient demographics and clinical characteristics1
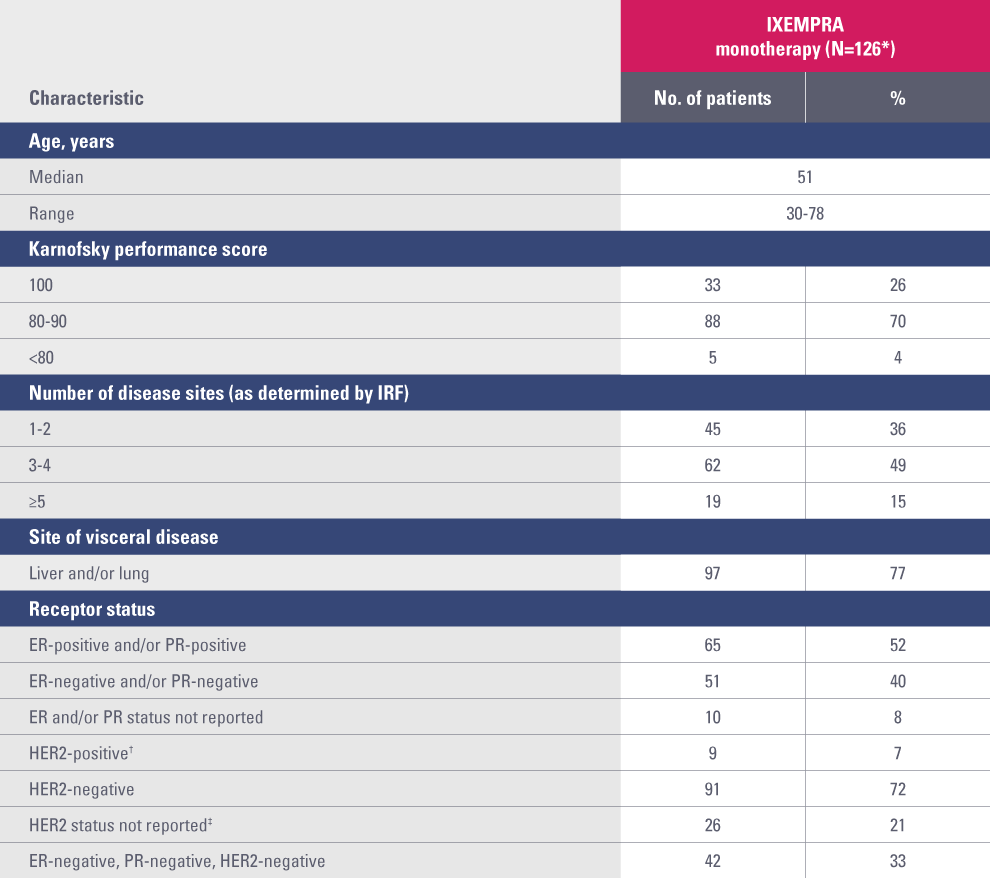
ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; IRF, independent radiology facility; PR, progesterone receptor.
* Two of the 128 patients enrolled were not treated: elevated total bilirubin precluded treatment in one patient, and the other refused treatment.
† Defined as positive by fluorescence in situ hybridization and/or 3+ by immunohistochemistry.
‡ HER2 testing was not performed routinely in sites in Latin America.
As monotherapy, IXEMPRA® (ixabepilone) demonstrated an objective tumor response rate of 12.4% and 18.3% (IRR vs IA, respectively)2
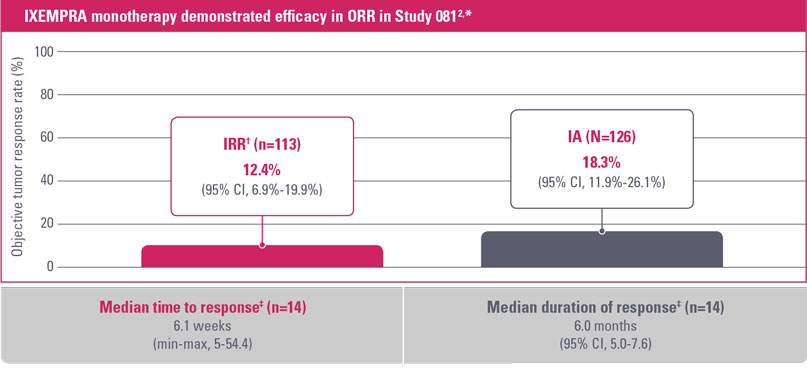
CI, confidence interval; IA, investigator assessment; IRR, independent radiologic review; ORR, objective tumor response rate.
* IXEMPRA was administered at a dose of 40 mg/m2 IV over 3 hours every 3 weeks. Patients received a median of 4 cycles (range, 1-18).
† All responses were partial.
‡ As assessed by IRR.
- Approximately 70% of patients received IXEMPRA after initial taxane failure3
- Objective tumor response rates2:
- IRR* (n=113): 12.4% (95% CI, 6.9%-19.9%)
- IA (N=126): 18.3% (95% CI, 11.9%-26.1%)
- Median time to response by IRR (n=14) was 6.1 weeks (min-max, 5-54.4)2
- Median duration of response by IRR (n=14) was 6.0 months (95% CI, 5.0-7.6)2
* All responses were partial.

Trina
Age: 45 years*
Perimenopausal
DIAGNOSIS
Initial stage
- Palpable mass in the right breast
- Mammography revealed 3.5-cm mass
- Ultrasound-guided biopsy confirmed presence of invasive ductal carcinoma
- Chest X-ray, bone scan, and CT scan were negative for metastatic disease
Receptor status
- ER−, PR−, HER2/neu−
TREATMENT HISTORY
- Underwent a mastectomy with axillary node dissection performed approximately 1 month after initial presentation
- 6 of 12 axillary nodes contained macrometastases, and 1 contained 2-mm micrometastasis (pT2N2)
Adjuvant
- Adjuvant therapy (AC–>T) initiated
- Patient subsequently received radiation therapy
PRESENTATION
General
- 9 months after completion of adjuvant therapy, patient complained of abdominal pain and shortness of breath on mild exertion; unable to work a full day due to severe fatigue
Sites of metastases
- Multiple liver and lung lesions
Pertinent labs
- CT-guided biopsy of one of the liver nodules was consistent with invasive ductal carcinoma
- Receptor status confirmed as ER−, PR−, and HER2/neu−
- LFT (AST/ALT) values were normal
- Neutrophil and platelet counts were within normal limits
FIRST-LINE METASTATIC CHEMOTHERAPY
- 16 months after surgery, patient was started on docetaxel plus capecitabine
- Further anthracycline therapy was contraindicated
- Patient progressed within 4 months

Safety profile of IXEMPRA® (ixabepilone) monotherapy2
Study 081 peripheral neuropathy

* Sensory and motor neuropathy combined.
- Prior therapy with neurotoxic chemotherapy agents did not predict the development of neuropathy
- Peripheral neuropathy was common and primarily sensory
- Neuropathy is cumulative and generally reversible and should be managed with dose adjustments and delays
- The median time to improvement of grades 3/4 neuropathy to baseline or grade 1 was 4.6 weeks
- Following dose reduction, 87% of patients had improvement or no worsening of their neuropathy
- Discontinuation due to neuropathy was 6%
Study 081 hematologic abnormalities
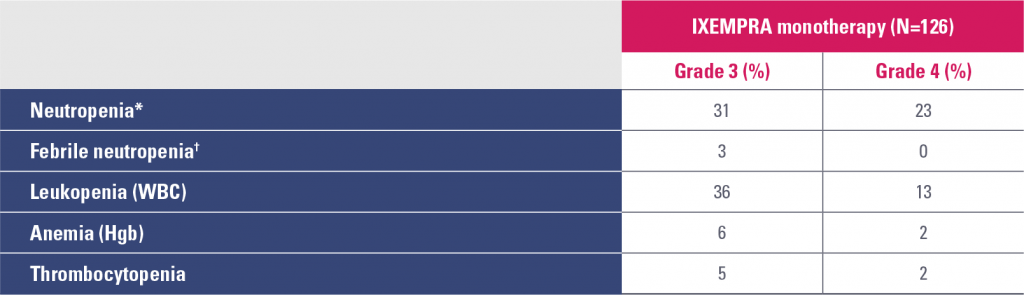
Hgb, hemoglobin; WBC, white blood cell count.
* Granulocyte colony-stimulating factor (G-CSF) or granulocyte-macrophage colony-stimulating factor (GM-CSF) was used in 17% of patients who received IXEMPRA in Study 081.
† In clinical trials, febrile neutropenia was classified as a nonhematologic adverse reaction.
- Infection with neutropenia was reported in 5% of patients treated with IXEMPRA as monotherapy
Study 081 nonhematologic drug-related adverse reactions occurring in ≥5% of patients
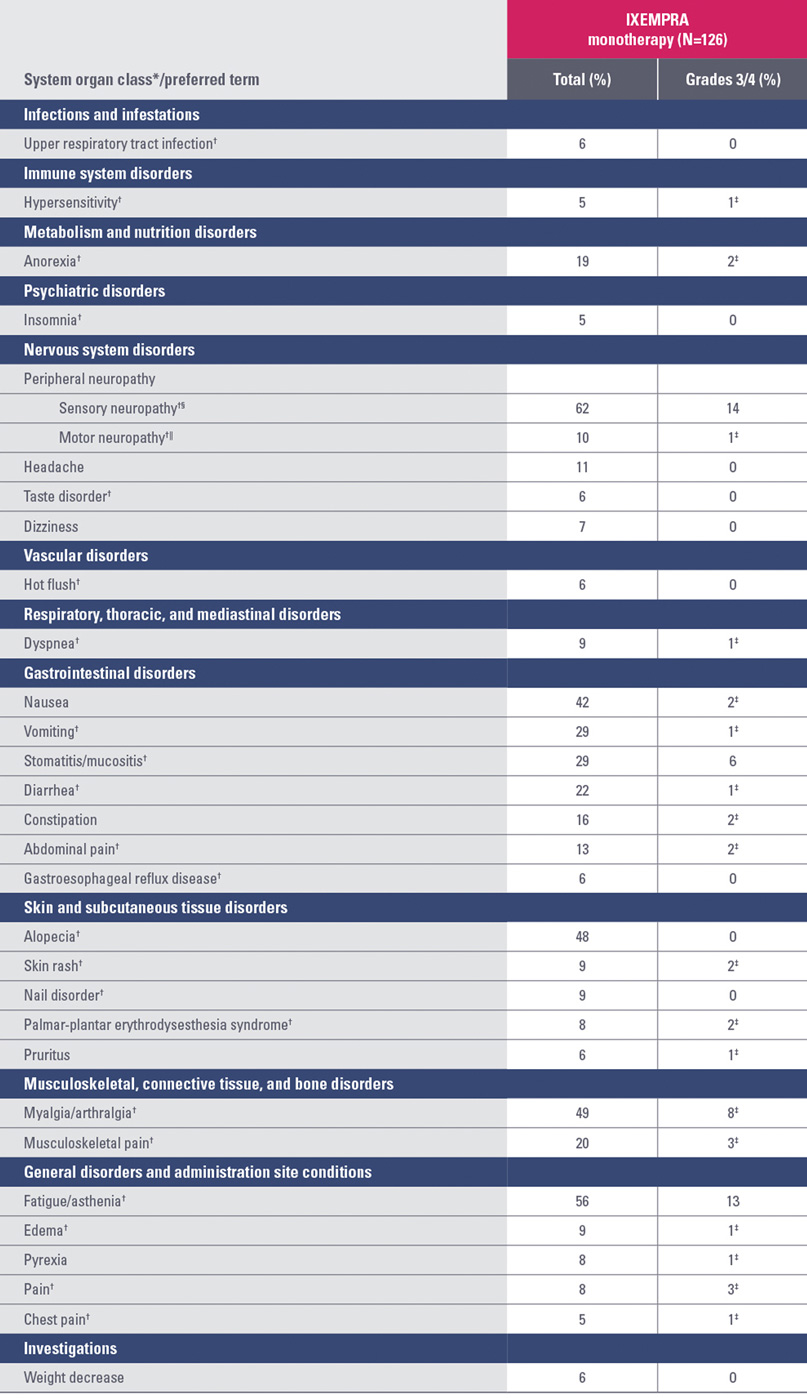
* System organ class presented as outlined in Guidelines for Preparing Core Clinical-Safety Information on Drugs by the Council for International Organizations of Medical Sciences (CIOMS).
† A composite of multiple MedDRA Preferred Terms.
‡ No grade 4 reports.
§ Peripheral sensory neuropathy (graded with the National Cancer Institute Common Terminology Criteria [NCI CTC] scale) was defined as the occurrence of any of the following: areflexia, burning sensation, dysesthesia, hyperesthesia, hypoesthesia, hyporeflexia, neuralgia, neuritis, neuropathy, neuropathy peripheral, neurotoxicity, painful response to normal stimuli, pallanesthesia, paresthesia, peripheral sensory neuropathy, polyneuropathy, polyneuropathy toxic, and sensorimotor disorder.
|| Peripheral motor neuropathy (graded with the NCI CTC scale) was defined as the occurrence of any of the following: multifocal motor neuropathy, neuromuscular toxicity, peripheral motor neuropathy, and peripheral sensorimotor neuropathy.
References: 1. IXEMPRA (ixabepilone) Prescribing Information; January 2016. 2. Perez EA. Microtubule inhibitors: differentiating tubulin-inhibiting agents based on mechanisms of action, clinical activity, and resistance. Mol Cancer Ther. 2009;8(8):2086-2095. 3. Yardley DA. Drug resistance and the role of combination chemotherapy in improving patient outcomes. Int J Breast Cancer. 2013;2013:137414. doi:10.1155/2013/137414.
